Research Suggests Path From Waste Plastics to High Value Composites
While most raw materials decline in availability over time, waste plastic reserves seem poised to increase for the foreseeable future. Research conducted at Rice University suggests that this resource could be converted to high value nanomaterials.
In a 2020 paper, a Rice research team described how its flash joule process was able to synthesize graphene using various plastics, including post-consumer HDPE. Now, they have shown it can convert HDPE and even unsorted, unwashed plastic waste into carbon nanotubes.
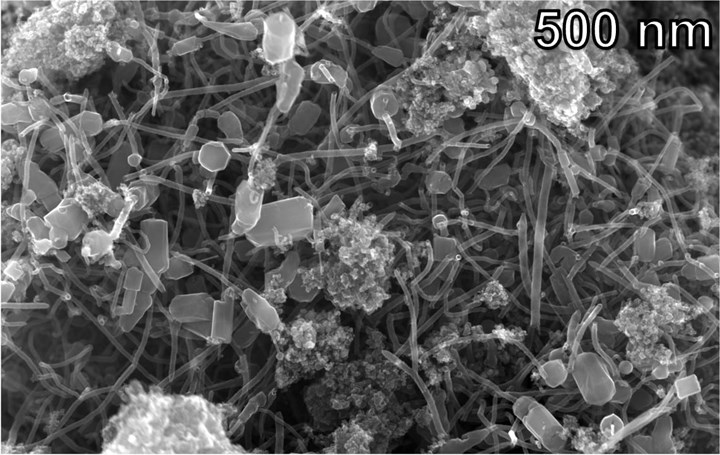
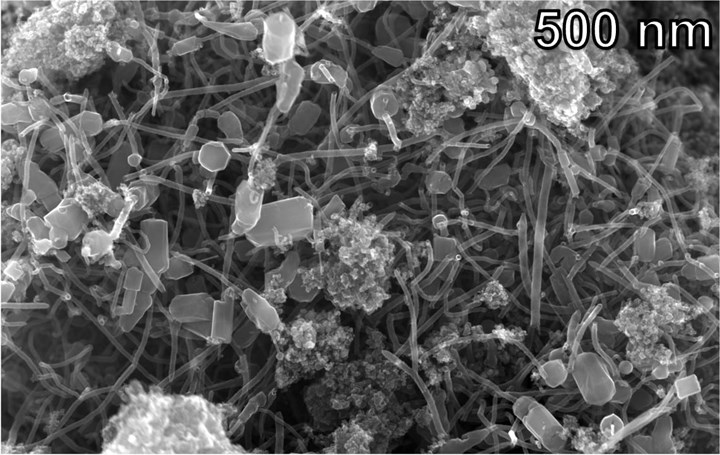
Hybrid carbon nanomaterial, consisting of nanotubes with bits of graphene attached to their ends, obtained from mixed waste plastics.
Photo Credit: Tour lab/Rice University
Graphene is an atom-thick layer of hexagonal carbon lattice, the familiar material graphite consists of many layers stacked together. Carbon nanotubes can be visualized as that hexagonal lattice rolled into a tube. They are the world record holder for ultimate tensile strength, individual nanotubes have been experimentally tested up to 63 GPa. They are also lightweight, have high surface area and are chemically robust.
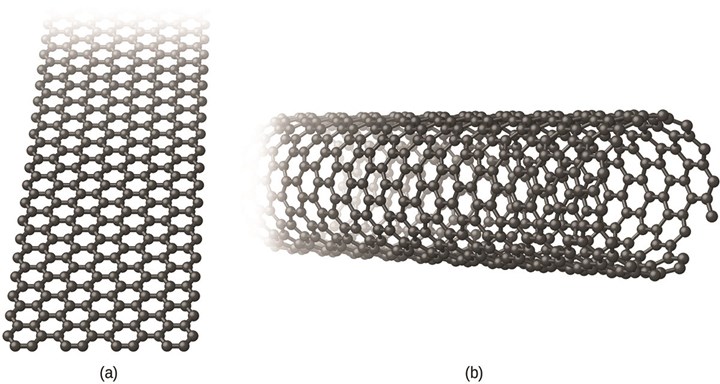
An illustration of the structure of graphene (a) and a single layer carbon nanotube (b).
Photo Credit: OpenStax (CC by 4.0)
These incredible properties have not translated to miraculously strong macro-scale structures, but carbon nanotubes have successfully been dispersed in cements, resins, coatings, and polymer melts, lending conductivity, strength, and thermal stability to composite materials.
Manufacture of carbon nanotubes by existing methods can be costly and energy-intensive, however, limiting the applications that have reached the market thus far. Arc discharge requires graphite as a raw material and several purification steps. Chemical vapor deposition can use plastic feedstock but requires high purity gases and growing the nanotubes takes several hours at elevated temperatures.
Flash Joule Heating for Nanotube Synthesis
According to the lifecycle analysis conducted at Rice, the flash joule heating process uses 86-92% less energy and produces 92-94% less greenhouse gas emissions compared to commercial methods. The process involves heating carbon feedstock rapidly using an electrical current. It requires no solvent or water, and prior work has shown an electricity cost of only $500 per ton of material.
Flash joule heating produces carbon nanomaterial in powder form. Researchers found that when added to epoxy resin, the resulting composite materials outperformed those made with commercially available graphene or carbon nanotubes. Bits of graphene are formed at the end of the nanotubes, and researchers believe this structure is responsible for the improved performance. Volatile hydrocarbons are also produced by the process, these will be studied to determine commercial value.
Using different metal catalysts and voltages, the researchers found that they could adjust the properties of the produced material. “Being able to control the shape and size of the carbon nanotubes produced was very exciting,” says Kevin Wyss, lead author of the new research paper, which has been accepted by the journal Advanced Materials.
The researchers are encouraged by the results to study additional ways to use the technology and are enthusiastic about the possibilities. “We are working to study what other types of materials and useful morphologies we can make using this flash joule heating strategy!,” says Wyss.
A startup company, Universal Matter, has formed to scale up flash joule heating as a production process, and plans to start a demonstration plant making graphene later this year.

Leave a Reply