Bringing Recycled Film into Food Packaging
Food packaging is a growing segment of the plastics market but the need for controlling safety has been one challenge limiting recycling rates. The existence of a market for high quality rPET and rHDPE has likely been a factor in the higher recycling rates relative to other plastics such as flexible food packaging. In 2018, the EPA estimated recycling rates of 29.1% and 29.3% of generation for PET bottles and natural HDPE containers, respectively, compared to an overall rate of just 13.6% for plastic containers and packaging in general.
Gaining FDA Approval, or Rather, “No Objection”
For recyclers aiming for the food packaging market in the U.S., obtaining an FDA “letter of no objection” is critical. The FDA reviews the processes a company proposes to use to collect and recycle materials to make new food contains. The letter of no objection indicates that the processes have been determined to be effective and identifies conditions of use that are appropriate for the material.
“Historically, many recycling processes submitted to the FDA for review have focused on PET. However, as the overall trend of utilizing recycled content in packaging has increased, the FDA has seen an increase in submission for additional recycled plastic resin types in food packaging,” says an FDA spokesperson.
Successful submissions demonstrate clearly that a recycler has source and process control systems in place to ensure the product will be food safe. “Really understanding your process and being able to articulate that back to the FDA is very important,” says David Hudson, chief strategy officer at Circulus. Circulus obtained a letter of no objection for recycled LDPE in December, fulfilling a goal since the company’s founding in 2019.
An important aspect of a recycling process control is control of the input stream. For Natura PCR, which also received an FDA letter of no objection in December (for its LLDPE recycling process) the key was identifying feed streams that were not coming from MRFs or other single-stream collection operations, where contamination with other materials could cause inconsistent quality.
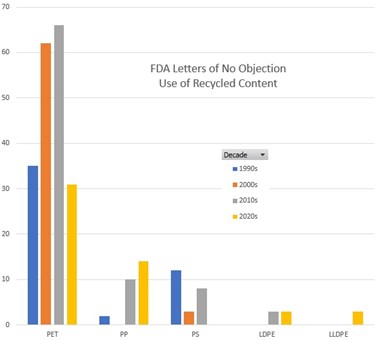
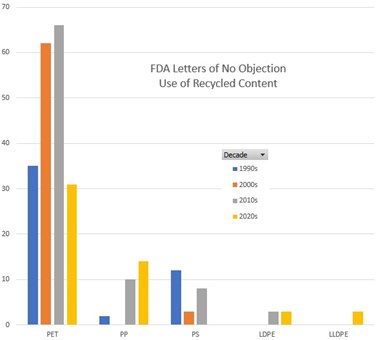
FDA has sent letters more for PP, LDPE, and LLDPE in the past few years, and fewer for PET and PS.
Credit: Derived from FDA data.
The letters received by both companies covered the use of recycled materials under conditions of use E through G, which are; room temperature filling and storage, refrigerated storage, and frozen storage. The letters were among 27 issued in 2022, an all-time high. If the polymers covered by these letters indicates a trend, it is that recyclers are interested in marketing a widening diversity of materials in the food packaging segment, well beyond the traditional PET bottle to bottle model.
Recycling Challenges in Food Safe Soft Flexible Packaging Materials
PET bottles and HDPE jugs have compact form factors that ease sorting, are of relatively uniform composition, and are processed at high temperatures, making them favored recycling targets historically.
“There are a lot of inherent challenges with film recycling that aren’t necessarily encountered by those who recycle rigids,” says David Hudson. “There’s a challenge putting it through a process in a highly consistent manner. Also, collecting large volumes of postconsumer film is very difficult in comparison to that of rigid plastics, which already have collection networks.”
One challenge with film packaging is simply its low weight. “It takes a lot of film to make just one bale” observes Jon Stephens, CEO at Natura PCR. “Some companies say ‘look, it’s just not worth it from a handling and labor standpoint’ so we’ll see what it takes to change these mindsets. We feel there is a lot of opportunity to capture more materials.”
The Opportunity for Film Recyclers
Despite the challenges, recyclers are optimistic about the opportunity for food grade recycled packaging and encouraged by the FDA letters.
“By obtaining the FDA LNO we’re able to go into a much broader range of applications and having our products end up in packaging that goes back on a store shelf,“ says David Hudson. Circulus plans to obtain additional letters of no objection covering operations at its future facilities.
Natura PCR hopes to broaden the conditions of use for its materials, and to bring in other types of recycled materials.
“We chose this route because we knew we could get good source material, and we wanted to make sure we can get the first letter and commercialize the product. From there, we plan to expand the conditions of use for that product, and get approval for additional products,” says Stephens. “There’s tremendous runway to grow this market segment. But overall, with plastics—especially in the US—we definitely need to improve the infrastructure and accessibility for recycling, we need to improve collection rates,“ says Stephens.
A 2020-21 report from the Sustainable Packaging Coalition found that 31.9% of US residents had access only to drop-off recycling (no curbside), and 8.6% had no access at all. Drop off programs exist for plastic film, but curbside collection is rare.
“As a nation we can definitely move the needle. Just think of the impact we can have on the industry and on the environment, if we went from just 30% on bottles to 50%, if we went from just 10% on film to 25. We have to continue to work together across the industry, all stakeholders, on how we communicate that to consumers in our community, “ says Stephens.

Leave a Reply